Google Ads
Module 1.1
Conductors & Insulators
- After studying this section, you should be able to:
- • Recognise common electrical conductors and their uses.
- • Recognise common electrical Insulators and their uses.

Conductors
In electrical and electronic circuits and components, CONDUCTORS are materials that allow electric current to flow through them because their atomic structure lets the outermost (free) electrons move easily from one atom to another, and because the electrons carry a negative electric charge they are easily repelled by a applied negative electric charge and attracted by a positive charge. Therefore applying a voltage between the two ends of a conductor causes an ‘electron drift ’ from negative to positive giving rise to an electric current. Suitable materials to act as conductors include:
Most Metals
Some Gases
Solutions of Acids, Alkalis & Salts in water
Metal Conductors

Metals such as copper, aluminium, and some alloys (mixtures of two or more metals), e.g. brass, phosphor-bronze and manganin are widely used in electrical and electronic circuits. Electric connectors in switches or in a mains 13A plug use a good conductor such as brass for their main contacts. Phosphor bronze, an alloy of copper and tin with some phosphorous, is springy in nature and useful for contacts such as the fuse holder in a mains plug, as well as "brushes" used to carry current between the stationary and rotating parts in some electric motors.
The best metal conductor of all is pure silver; however it has two drawbacks. Its surface readily tarnishes when exposed to air, this creates a high resistance surface that reduces its conductivity. Although this doesn't cause a great problem in high voltage conductors, it can significantly reduce conduction in low voltage applications such as switch or plug/socket contacts. Silver is also expensive compared to other metals such as copper (the second best metal conductor). Although Copper is the most popular metal for electrical conductors,it also tarnishes, so copper conductors are often plated with less tarnishable metals such as nickel. For optimum contact conduction in low voltage applications however, gold plating is used because, although slightly less conductive than pure copper, gold does not tarnish.
Gold plating is commonly used to reduce 'skin effect' in conductors used for high frequency current applications. Skin effect is the tendency of high frequency currents to flow mainly close to the surface of the conductor. Using gold plating therefore provides a current path with a resistance only slightly worse than copper in the body of the cable, but considerably better that the tarnished outer layer of the cable or connector. Hence it is common to see gold plated sockets and connectors in devices such as mobile phones and even audio equipment.
Manganin is another copper-based alloy but with higher resistance, used in the construction of wire wound resistors and heater elements for high power applications.
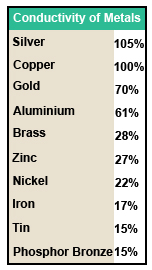
Fig. 1.1.1 Conductivity of Metals
Aluminium is commonly used as a conductor. Though not as good a conductor as copper, it is much lighter and useful for applications such as the large power distribution cables crossing the landscape strung on electricity pylons. Copper cables would be too heavy for this job. Aluminium also has some properties, useful in the construction of transistors and diodes.
Silver is an even better conductor than copper, but since it is more expensive, it is only used in very small amounts.
The conductivity of metals used for electrical purposes may be compared to an internationally agreed standard value, as shown in Fig 1.1.1. That of annealed copper, that is copper that has been heated and cooled at a controlled rate to soften the metal and remove any stresses present. Other commonly used metals can therefore be compared to this standard where a 100% rating indicates the same conductivity as copper, over 100% indicates a better conductor and less that 100%, a worse conductor. Note that the table only compares standard samples of metals and does not take into consideration effects due to tarnishing or different frequencies of current etc.
Conductive Gases

Some gases can pass current, such as neon for example, which produces a typical orange glow when a small electric current is passed though it at a high voltage. Neon indicator lamps have many uses and can be used with either AC or DC current.

In neon and fluorescent lighting, colours other than orange can be produced by adding gases such as argon, mercury, or helium at low pressure. Light is produced by applying a high voltage between two electrodes at either end of the tube, which causes the gas molecules to ionise and so emit photons, giving light either directly through the clear glass tube, or indirectly by exciting a phosphor coating on the wall of the tube to give a greater range of colours.
Insulators
Materials that prevent the flow of electric current are called INSULATORS. These are mostly solid materials in which the outer electrons of each atom are tightly bonded to the nucleus of the atom, preventing any electron movement within a ‘normal range’ of applied voltage. Materials commonly used in electronic circuits include:

Plastics (e.g. Polystyrene, PVC Bakelite and Polythene)
Glass (including Fibre Glass)
Ceramics
Resins (e.g. epoxy resins)
Paper (usually impregnated with wax, resin etc.)
Rubber (Natural or synthetic)
Air
Both the terms "Insulator" and "Conductor" are relative. That is, they each have some properties of the other as well as their own. For example an insulator may pass very small currents, but not sufficiently to be called a conductor. An insulator can work well at low voltages, such as those found in battery operated equipment, but fail totally and pass large currents if connected to a much higher voltage.


